Ensuring High Performance of Sensitive Laboratory Equipment
27 Feb. 2020

Purified Water – Providing the Right Purity is Critical in this Key Reagent for Laboratory Equipment
Chemical and Analytical lab instruments are very dependent on the quality and reliability of the lab supplies available. In particular, the impurities in the purified water used will have a critical effect on laboratory equipment performance. Clearly, different techniques vary in their sensitivity to water purity. Many of the issues are straightforwardly predictable e.g. if you plan to measure trace sodium, negligibly low concentrations of sodium in your pure water would be a very good idea.
However, others are less obvious: when counting bacteria, bacteria-free water would also be obviously helpful but the organic content of the water can also markedly affect growth rates and so alter the number of countable colonies. Similarly, in ICP-MS for trace metal determination, organics in the water can seriously interfere with the results.
The table below gives general indications of the significance of the main categories of water impurities for a number of lab instruments and other lab techniques.
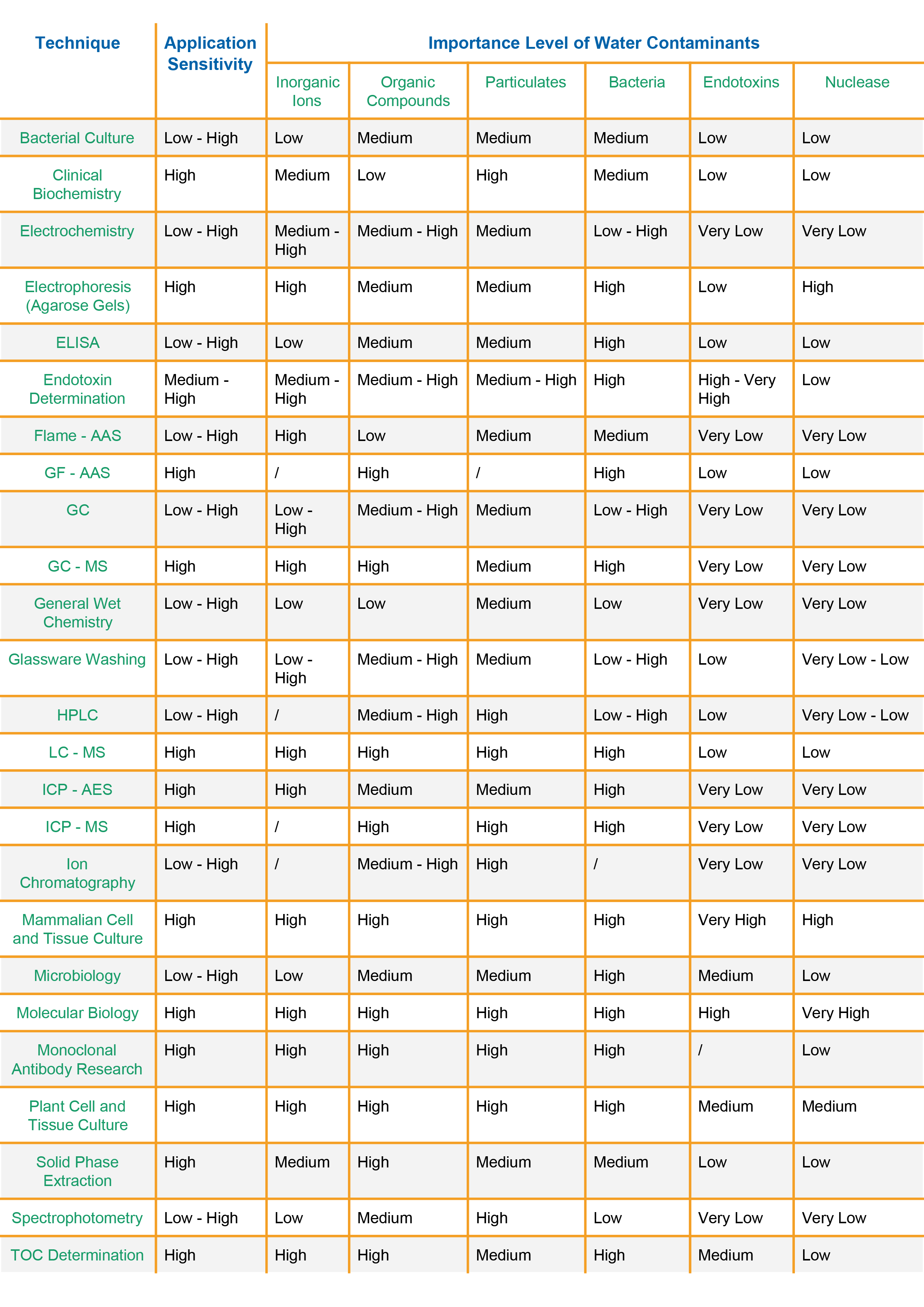
Application sensitivity refers to the concentration of the analyte being measured: typically high sensitivity = ppb or lower, medium = ppb to ppm and low = ppm or higher. The data in the table is taken from “ISPE Baseline Pharmaceutical Engineering Guide, Volume 4, Water and Steam Systems, 2011”. Numeric impurity values are not given due to the variation in water purity needed within any one type of application.
Ensuring Your Supply of Purified Water Will Continue To Meet Your Lab Instrument Needs
The impurities in the lab supply of purified water will have a critical effect on laboratory equipment performance. It is, therefore, essential to install a lab supply that provides water with low enough impurity levels, to maintain it correctly and to monitor it to ensure that the water purification equipment is working correctly.
You should look to install a system that is designed to be robust:
1) is resistant to known issues such as bacterial growth
2) will effectively and rapidly detect changes in organic and ionic contamination and
3) will effectively and rapidly detect the need to change purification media.
Robustness is a feature of laboratory equipment design. Bacterial control can be achieved using a 0.2-micron point-of-use (POU) filter but relying on such a filter alone is not robust.
Bacterial contamination can build up within the unit and the filter can become overloaded or fail unpredictably ruining laboratory experiments using the water for preparation or testing.
A robust water purification system design will incorporate some or all of the following: carbon filtration and reverse osmosis to reduce the overall level of organic and bacterial contamination, recirculation to minimize static water and the growth of biofilm, exposure to 254nm UV light to deactivate residual bacteria and, finally if needed, a POU filter.
Such a system should also be easy to sanitize if required. This is, clearly, more complicated and more expensive but, ultimately, much more reliable and will also minimize levels of endotoxin and nuclease.
Unlike bacterial contamination, the presence of ionic and organic impurities can be monitored easily online. Electrical conductivity provides a continuous and straightforward indication of total contamination from ions at levels above about 1ppb.
Total organic contamination can also be monitored down to <1ppb C by on-line TOC. However, unlike conductivity cells, TOC monitors differ in their modes of operation and it is essential to use one with a rapid response time.
Contamination in purified water can build up over time, e.g. due to biofilm growth, or rapidly due, for example, to release of weakly bound organic or ionic species from over-loaded purification media or the loss of a purification process due to a UV lamp or filter failure.
The objective of monitoring is not to check that the contaminants are at a low enough level to be used in any particular lab experiment or analysis; it is to check that the water purifier is working normally i.e. that the purity parameters of conductivity and TOC are normal.
If they have deteriorated then there could be an issue and it needs to be investigated before more water is used.
A Lab Supply of Purified Water – As Much or as Little as You Need
Lab supplies of purified water are available in a variety of quantities and qualities, from ultrapure water to biopure. The type of water purifier required is dictated by the applications, the types of laboratory equipment being used, regulatory requirements, e.g. the US or European Pharmacopeia, and the proportions of different water purities needed.
With a single piece of laboratory equipment requiring high purity water, the choice is likely to be between a single dedicated stand-alone unit supplied with mains water and suitably designated or verified bottled water.
Most labs these days have a number of laboratory instruments and scientific applications requiring the supply of much larger quantities of purified water, often with different purities.
There are, then, a number of choices available. If you have the luxury of a new building being built, then, you can consider all the options; in other cases, practicalities and costs will restrict choice:
1) A “central” purification system with a permanent distribution system to the entire building
2) One or more semi-localized purification systems with local distribution loop to a lab or floor
3) Small purification system with local storage and loop
4) Individual point-of-use units supplied with mains water
5) An extension of a distribution system used for another purpose, such as manufacturing
6) Water polishers or dispensers fed from any of these.
Of the first four general options, Option 1 offers advantages of scale and central monitoring but is less flexible to meet changes in application and the site supply is vulnerable to system break-down. It is most suitable where an equivalent water quality is required in a variety of locations. On-going maintenance requires the availability of a trained technician.
Options 2 and 3 maintain some advantages of scale, are more flexible and if one system goes down water may be sourced from another system. They also have the possibility of circulating the lower level of water purity needed, say for glass-washing, and using this to supply polishers for those lab instruments requiring higher purities. Lab staff can be trained to carry out routine maintenance.
Option 4 is the most flexible option; it is easiest to adapt to changes and different units can be used for different applications. It is also the easiest to install. Common consumables can offset the cost of multiple units. Users can take responsibility for routine checks and consumable replacement with the advantage of local “ownership”. There are a number of sites with over 50 such POU units.
The above choices are real viable alternatives and give the user plenty of opportunities to ensure the highest quality of water is used in their sensitive laboratory equipment.
Find out more about ELGA’s Laboratory Water Purification Systems and how they can ensure the high performance of your laboratory equipment
Dr Paul Whitehead
After a BA in Chemistry at Oxford University, Paul focused his career on industrial applications of chemistry. He was awarded a PhD at Imperial College, London for developing a microwave-induced-plasma detector for gas chromatography. He spent the first half of his career managing the analytical support team at the Johnson Matthey Research/Technology Centre,specialising in the determination of precious metals and characterising applications such as car-exhaust catalysts and fuel cells. Subsequently, as Laboratory Manager in R&D for ELGA LabWater, he has been involved in introducing and developing the latest water purification technologies. He now acts as a consultant for ELGA.
